Background:
C-CAR088, an anti-BCMA CAR T-cell therapy is a novel 2nd generation 4-1BB chimeric antigen receptor T (CAR-T) cell therapy targeting BCMA which is specifically and highly expressed on multiple myeloma (MM) cells. C-CAR088 is manufactured in a serum-free, automated and digital, closed system. Initial, early clinical trial results in patients with R/R MM supported preclinical findings and showed promising efficacy and manageable safety profile (Yao, Blood (2019) 134 (Supplement_1): 50.)
Methods:
The dose escalation and expansion studies have been conducted at four medical centers in China to evaluate the safety and efficacy of C-CAR088 in patients with R/R MM who were previously treated with at least 2 lines of therapy including proteasome inhibitors (PIs) and IMiDs. C-CAR088 is administered to patients as a single intravenous dose after a standard 3-day cyclophosphamide/fludarabine conditioning regimen.
Results:
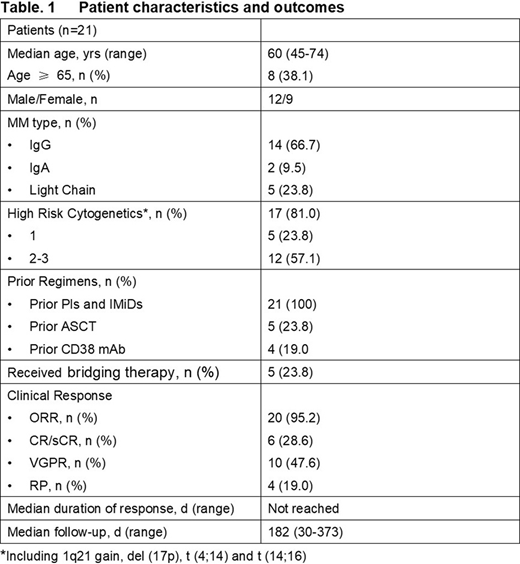
As of July 15, 2020, 24 patients were infused and 21 patients had evaluable data for safety and clinical response at dose levels of 1.0 x 106 CAR-T cells/kg (n=3), 3 x106 CAR-T cells/kg (n=11) and 4.5~6x106 CAR-T cells/kg (n=7). The median vein to vein time was 16 days. The manufacturing success rate was 100%. The median age of patients dosed was 60 years (range: 45-74 years).The median number of prior lines of therapy was 4 (range: 2-12 prior therapies). There were 17 (81%) patients with at least one and 12 (57.1%) patients with at least two high risk cytogenetic tumor changes. Five patients (23.8%) had bridging therapy.
C-CAR088 treatment was well tolerated. 20 of 21 (95%) patients had Grade 1-2 CRS and one patient experienced Grade 3 CRS. Median time to CRS was 6.5 days (range: 1-11 days) and median duration of CRS was 5 days (range: 2-10 days). Four patients (19%) received tocilizumab for CRS treatment. Only one patient experienced a Grade 1 neurotoxicity event. No dose-limiting toxicities were observed and all adverse events were reversible. The best overall response (BOR) included 6 complete responses (CRs), 10 very good partial responses (VGPRs) and 4 partial responses (PRs). Median follow-up was 182 days (range: 30-375 days). The median duration of response has not been reached. In the 3 x106 CAR-T cells/kg dose group, 5/11(45%) patients achieved a CR. The C-CAR088 PK profile in peripheral blood showed a trend of a dose dependent profile. AUC0~28day and Cmax increased and Tmax decreased with dose (P<0.05).
Conclusion:
The clinical trial results in patients with R/R MM treated with C-CAR088 show a favorable safety profile and promising signs of efficacy. We will continue to evaluate these patients to understand the long-term effect of C-CAR088 in multiple myeloma patients.
Clinical trial information: NCT04322292、NCT03815383、NCT03751293、NCT04295018
Research Sponsor: Cellular Biomedicine Group, Inc.
Zhu:Cellular Biomedicine Group Inc: Current Employment, Current equity holder in publicly-traded company. Zheng:Cellular Biomedicine Group Inc: Current Employment, Current equity holder in publicly-traded company. Yan:Cellular Biomedicine Group Inc: Current Employment, Current equity holder in publicly-traded company. Lv:Cellular Biomedicine Group Inc: Current Employment, Current equity holder in publicly-traded company. Lan:Cellular Biomedicine Group Inc: Current Employment, Current equity holder in publicly-traded company. Yang:Cellular Biomedicine Group Inc: Current Employment, Current equity holder in publicly-traded company. Huo:Cellular Biomedicine Group Inc: Current Employment, Current equity holder in publicly-traded company. Han:Cellular Biomedicine Group Inc: Current Employment, Current equity holder in publicly-traded company. Zhao:Cellular Biomedicine Group Inc: Current Employment, Current equity holder in publicly-traded company. Qin:Cellular Biomedicine Group Inc: Current Employment, Current equity holder in publicly-traded company. Wu:Cellular Biomedicine Group Inc: Current Employment, Current equity holder in publicly-traded company. Yao:Cellular Biomedicine Group Inc: Current Employment, Current equity holder in publicly-traded company. Zhu:Cellular Biomedicine Group Inc: Current Employment, Current equity holder in publicly-traded company. Ren:Cellular Biomedicine Group Inc: Current Employment, Current equity holder in publicly-traded company. Zhang:Cellular Biomedicine Group Inc: Current Employment, Current equity holder in publicly-traded company. Huang:Cellular Biomedicine Group Inc: Current Employment, Current equity holder in publicly-traded company. Humphries:Cellular Biomedicine Group Inc: Current Employment, Current equity holder in publicly-traded company. Yao:Cellular Biomedicine Group Inc: Current Employment, Current equity holder in publicly-traded company.
Author notes
Asterisk with author names denotes non-ASH members.